Wir laden Fachpersonen, die in Zentren, Kliniken, Spitälern oder anderen Organisationen Patientinnen und Patienten mit seltenen Krankheiten betreuen, dazu ein, die Betroffenen über das Schweizer Register für seltene Krankheiten (SRSK) zu informieren.
Die nachfolgende Seite ist aktuell nur in Englisch verfügbar.

Conditions for SRDR Registration
If you think a patient should be included in the SRDR, please
- check their eligibility criteria,
- indicate their Orphacode, and
- obtain their informed consent.
Eligibility criteria
For inclusion in the SRDR there must be a high suspicion or a confirmed diagnosis of rare disease. The person must live, or must be treated for the rare disease in Switzerland. A comprehensive list of rare diseases can be found on Orphanet, the online portal for rare diseases and orphan drugs. The SRDR also records people retrospectively.
Orphacode
The registration in the SRDR requires rare disease coding by Orphacode. An Orphacode is a unique and permanent rare disease identifier developed and consistently updated by Orphanet. Other current disease classification systems, such as the International Classification of Diseases (ICD-10), lack specific codes for most rare diseases. As a result, rare diseases are inadequately represented in health statistics. The SRDR Steering Board established an Orphacode working group. If you have any questions about Orphacodes, such as practical coding with Orphacodes or their implementation in hospitals or other health care institutions, please contact Marlies Morf, (marlies.morf@insel.ch) who leads the Orphacode working group.
Informed Consent
The SRDR is subject to the Human Research Act (HRA) and to the Data Protection Act. The SRDR collects identifying data, which is why registration requires the patient’s informed consent.
Version of Informed Consent forms
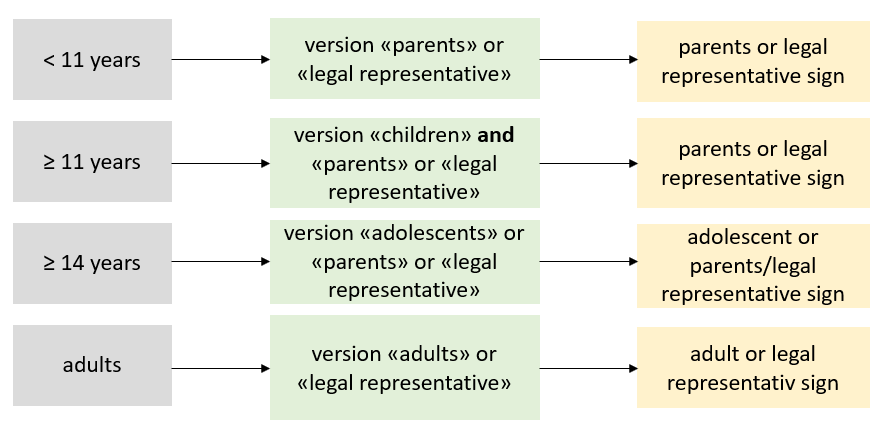
Informed consent forms are available for download in French, German, and Italian. There are 5 different, age- and situation-specific versions (regardless of the rare disease) with 2 different options for obtaining informed consent.
The 5 age- and situation-specific versions involve:
- children ≥ 11 years
- adolescents ≥ 14 years
- adults
- parents
- legal represenative
The 2 options for obtaining informed consent include either
- option A: the participant or their legal representative sign, or
- option B:the treating physician and the participant or their legal representative sign.
Use the option that best suits your procedure for obtaining informed consent. For example, it makes most sense for patient organizations to use option A where only the participant or their legal representative sign, which can minimize administrative work.
The figure below shows when you must hand over which version. If you have any questions about the different versions, please contact us.
Where to send the signed Informed Consent
Patient organizations should instruct participants to send the signed informed consent form to
Universität Bern
Institut für Sozial- und Präventivmedizin
Schweizer Register für seltene Krankheiten
Universität Bern
Mittelstrasse 43
3012 Bern
Participants who are recruited at centers, clinics, or hospitals should return the signed informed consent form to the original recruiting location.
The SRDR Dataset
Whether a person consents or declines to participate determines which 1 of 2 patient datasets will be included in the SRDR.
- If a person consents to SRDR registration, their Core Dataset is collected.
- If a person declines SRDR registration, an anonymous Minimal Dataset is collected.
Core Dataset when a person consents to SRDR inclusion
The SRDR Core Dataset is a slim dataset collected for all rare diseases. It corresponds to the European Union’s (EU) Rare Disease Platform «Set of common data elements for Rare Disease Registration» to maximize interoperability between rare disease registries.
The following information is collected for patients who consent to participate
- patient information (e.g., name, address, date of birth)
- informed consent (e.g., signed, date of signature)
- patient status (e.g., vital status)
- clinical data (e.g., Orphacode, diagnostic method, age ate first occurrence of symptoms)
- if patient is registered in another registry
- treating institution (e.g., name of treating hospital)
We assembled a comprehensive list of the Core Dataset variables recorded in SRDR.
Minimal Dataset when a person declines SRDR inclusion
The SRDR Minimal Dataset only collects unidentifiable variables. To achieve data completeness, we need to include a Minimal Dataset. If we do not record Minimal Datasets for those who decline participation, we will not have an accurate representation of rare diseases visible in health statistics.
The following anonymous information is collected for patients who declines to participate
- sex, year of birth
- informed consent status (e.g., refused, withdrawn)
- Orphacode
We assembled a comprehensive list of the Minimal Dataset variables recorded in SRDR.
Options for Data Transfer
There are 3 options to transfer datasets to the SRDR, which depend on
- the size of the clinic or practice and number of patients with rare diseases, and
- the existing method used to store patient data in the clinic or practice.

If you have any questions about transferring patient datasets, please contact us.
FAQ
Is it necessary to inform local ethics committees about data transfers to the SRDR?
Ethics Commission of the Canton of Bern (KEK Bern) approved the SRDR’s protocol as a monocentric study. The ethics approval is valid throughout Switzerland. The Institute of Social and Preventive Medicine and SwissRDL take full responsibility for data security and access. From the perspective of the SRDR, it is not necessary to inform local ethics committees; however, those who provide data should follow their clinic internal procedures for sharing patient data.
What contribution can the SRDR make to health policy?
For the first time in Switzerland, the SRDR will provide an accurate representation of people living with rare diseases, and descriptive statistics for rare diseases in general and for specific rare diseases.
Does the SRDR and the Coordination Rare Diseases Switzerland (kosek) cooperate with the European Reference Networks (ERN)?
Kosek aligns criteria for recognizing reference centers in Switzerland with criteria for participation in ERNs. For Switzerland, it is possible to be represented in ERN groups as supporting, non-voting members who can join meetings and activities.
Who can access the SRDR data for research purposes?
Those who provide SRDR data will have access to the data that they provide at any time. The SRDR plans to set up a dashboard where summarized data are available, such as the numbers of patients within a specific group of rare diseases and general data about age. If you are interested in executing a study, please find further information under Advanced SRDR Studies.
What contribution can the SRDR make for research?
The SRDR allows for conducting ancillary studies.
